Shape-Selective Catalysis by Zeolites :
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`text(Definition) :` The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis.
`=>` Zeolites are good shape-selective catalysts because of their honeycomb-like structures.
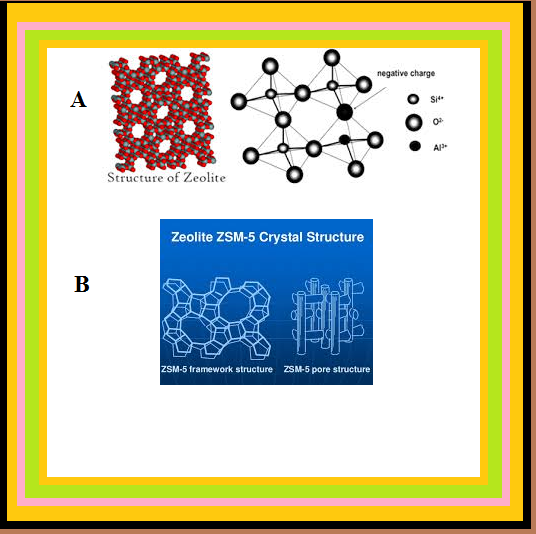
`=>` They are microporous aluminosilicates with three dimensional network of silicates in which some silicon atoms are replaced by aluminium atoms giving `Al–O–Si` framework.
`=>` The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites.
`=>` They are found in nature as well as synthesised for catalytic selectivity.
`=>` Zeolites to be used as catalysts are heated in vacuum so that the water of hydration is lost. Because of this zeolites become porous. The size of the pores varies between 260 pm and 740 pm. So only those molecules can enter these cavities whose size is small and can even leave easily.
`=>` So these zeolites act as selective adsorbents called molecular sieves.
`text(Uses of Zeolites :)`
● Zeolites are being very widely used as catalysts in petrochemical industries for cracking of hydrocarbons and isomerisation.
● An important zeolite catalyst used in the petroleum industry is ZSM-5.
● It converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
`text(Definition) :` The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis.
`=>` Zeolites are good shape-selective catalysts because of their honeycomb-like structures.
`=>` They are microporous aluminosilicates with three dimensional network of silicates in which some silicon atoms are replaced by aluminium atoms giving `Al–O–Si` framework.
`=>` The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites.
`=>` They are found in nature as well as synthesised for catalytic selectivity.
`=>` Zeolites to be used as catalysts are heated in vacuum so that the water of hydration is lost. Because of this zeolites become porous. The size of the pores varies between 260 pm and 740 pm. So only those molecules can enter these cavities whose size is small and can even leave easily.
`=>` So these zeolites act as selective adsorbents called molecular sieves.
`text(Uses of Zeolites :)`
● Zeolites are being very widely used as catalysts in petrochemical industries for cracking of hydrocarbons and isomerisation.
● An important zeolite catalyst used in the petroleum industry is ZSM-5.
● It converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.